Abstract
Background
Immunotherapy with anti-CD19 CART cells (CART19) induces complete remission (CR) in the minority of patients with CLL; however, these CRs tend to be durable (Porter Sci Tr Med 2015). Based on preclinical evidence of synergy, we combined anti-CD19 CAR T cells with ibrutinib to test the hypothesis that pre- and concurrent treatment enhances the CR rate.
Methods
This is a pilot trial of autologous anti-CD19 CAR T cells in adults with CLL/SLL who were not in CR despite at least 6 months of ibrutinib. T cells were lentivirally transduced to express a CAR comprising CD3z, 4-1BB, and humanized anti-CD19 scFv (CTL119). Pts underwent lymphodepleting chemotherapy up to 1 week before infusion, followed by planned infusion of 1-5x108 CTL119 cells dosed as 10%, 30% and 60% of the total planned dose over 3 days, with doses beyond dose#1 given only in the absence of fever or cytokine release syndrome (CRS).
Results
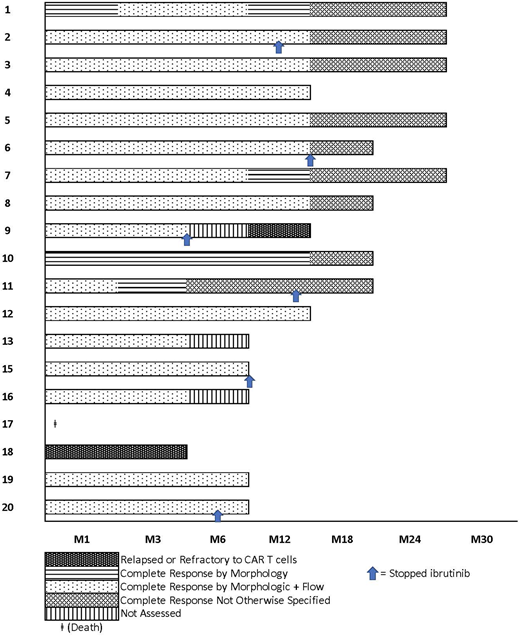
CTL119 manufacturing was successful for all pts. Twenty pts were enrolled and 19 were infused (one pt was not infused due to intercurrent large cell transformation and newly diagnosed adenocarcinoma). Of the 19, 15 were male, the median age was 62 (range 42-76); and 5 were on 1st line ibrutinib. Of the remaining 14, the median number of prior therapies was 2 (range 1-16), and 3 pts had received prior murine CART19 therapy (CTL019) without ibrutinib. Eleven pts had abnormalities of chromosome 17p or TP53. An additional 3 pts had abnormalities of chromosome 11q22 or ATM. All pts had marrow involvement (median 21% range 7-63%). For 9 pts who had enlarged nodes at baseline, the median cross-sectional area was 1471 mm2 (range 178-2220). All pts received at least 2 CTL119 doses; 14 patients received all 3 and 5 received 2 doses. The median number of CART cells given was 5.3x106/kg (range 2.0-7.5).
Median peak CART cell number by qPCR was 90,990 copies/ug genomic DNA (range 965-210,556) and by flow cytometry was median 536 CART cell/ul blood (range, 0-3640).
18/19 (95%) pts experienced CRS, with a median Penn CRS Grade of 2. CRS was grade 1-2 and 3-4 in 15 and 3 pts, respectively. Two pts received tocilizumab. Of 5 pts with encephalopathy, 2 were CTCAE gr 1, 2 gr 2 and 1 gr 4. One pt died on D14 from a cardiac arrhythmia during severe neurotoxicity after resolution of CRS. There were 49 gr 3 and 22 gr 4 toxicities in total.
As of 7/16/18, 18/19 pts are alive (95%) and 12 pts have been followed for at least 12 months. The median follow-up for the 18 surviving pts is 18.5 months (range 8-28). Per iwCLL criteria, at 3 months 14 pts were evaluable and their responses were CR (n=6), PR (n=4), SD (n=3), PD (n=1). Marrow responses at month 3 were available in 18 and showed a morphologic CR in 17 pts (94%); of these 15 also had no measurable residual disease (MRD) by 9-color flow cytometry. MRD was also assessed at 3 months by deep sequencing of the immunoglobulin heavy chain locus (limit of detection 1 B cell in 1x106 nucleated cells). 14/18 pts were MRD negative, and the remaining 4 had 3.36, 4.76, 1.79, and 0.48 log10 reduction of the leukemic clonotype relative to the baseline sample. LN biopsies from 2 pts 3 and 10 months after CTL119 confirmed absence of the CLL clonotypes in this compartment as well. At 12 months, 11 pts had evaluable marrows of which 10 (91%) were in morphologic CR and 1 showed morphologic relapse. Of the 10 in morphologic CR, three pts showed low MRD positivity (3.58, 2.34, 3.79 log10 reduction) and the rest remained MRD-ve. Of the 3 pts who had received murine CTL019 previously, 2 were in MRD+ve CR at 12 months and one was refractory to humanized CTL119.
Six pts discontinued ibrutinib at a median 8 months (range 3-12) due to toxicity (n=2) or pt choice (n=4). Five pts remain MRD-ve at short followup. In total, 16/18 pts remain in morphologic and/or flow CR at last followup.
Conclusion
In patients not achieving CR despite at least 6 months of ibrutinib who were treated with humanized CART19, we found an iwCLL CR rate of 43% and a bone marrow remission rate of 94% including a 78% MRD negative response by deep sequencing. This compares favorably to prior CART19 cell studies in patients with progressive CLL (iwCLL CR rates of 21-29%). CRS was frequent but mild-moderate and did not commonly require anti-cytokine therapy. These results suggest that the combination of CTL119 with ibrutinib results in a high rate of sustained responses and high rates of MRD-ve marrow response in patients with CLL. This combination will be further tested in larger studies.
Gill:Carisma Therapeutics: Equity Ownership; Extellia: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding. Frey:Servier Consultancy: Consultancy; Novartis: Consultancy. Mato:Johnson & Johnson: Consultancy; Portola: Research Funding; Acerta: Research Funding; AstraZeneca: Consultancy; AbbVie: Consultancy, Research Funding; Pharmacyclics, an AbbVie Company: Consultancy, Research Funding; Regeneron: Research Funding; TG Therapeutics: Consultancy, Research Funding; Celgene: Consultancy; Medscape: Honoraria; Prime Oncology: Honoraria. Lacey:Novartis Pharmaceuticals Corporation: Patents & Royalties; Tmunity: Research Funding; Parker Foundation: Research Funding; Novartis Pharmaceuticals Corporation: Research Funding. Melenhorst:Novartis: Patents & Royalties, Research Funding; Incyte: Research Funding; Tmunity: Research Funding; Shanghai UNICAR Therapy, Inc: Consultancy; CASI Pharmaceuticals: Consultancy. Davis:Novartis Institutes for Biomedical Research, Inc.: Patents & Royalties. Schuster:Gilead: Membership on an entity's Board of Directors or advisory committees; Physician's Education Source, LLC: Honoraria; Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Honoraria, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Nordic Nanovector: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Dava Oncology: Consultancy, Honoraria; Genentech: Honoraria, Research Funding; OncLive: Honoraria. Siegel:Novartis: Research Funding. Isaacs:Novartis: Employment. June:Immune Design: Membership on an entity's Board of Directors or advisory committees; Celldex: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceutical Corporation: Patents & Royalties, Research Funding; Tmunity Therapeutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Tmunity Therapeutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Immune Design: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceutical Corporation: Patents & Royalties, Research Funding. Porter:Kite Pharma: Other: Advisory board; Genentech: Other: Spouse employment; Novartis: Other: Advisory board, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal